Home > Solutions > Regulatory Compliance > Pharmaceutical Compliance
Systech offers powerful serialization and traceability functionality to help you meet global pharmaceutical regulatory compliance.
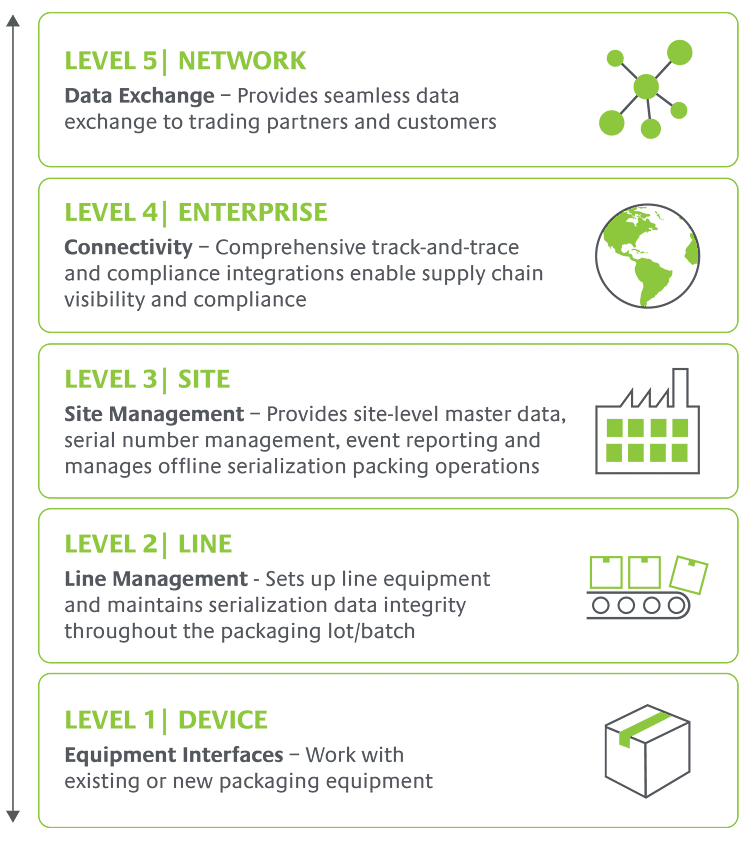
Our proven software platform covers the full stack—from Level 1 to Level 5—for unbeatable ease of implementation and speed to compliance. Equipment-agnostic, configurable solutions give you flexibility to adapt to new pharma regulatory compliance requirements cost-effectively without customizing for every change, wherever you do business.
Compliance mandates data accuracy which mirrors the physical product movement from point of manufacture to point of dispense. Our complete solution enables seamless and secure data exchange for traceability throughout the entire supply chain. This—combined with the ability to connect with master data systems and regulatory databases—creates a compliant system of record for your products.

A timely example is the final DSCSA milestone which mandates the implementation of an electronic, interoperable system to trace prescription drug products at the package level by November, 2024. These Enhanced Drug Distribution Security Requirements impact the entire pharma supply chain ecosystem: manufacturers, distributors, dispensers, repackagers, 3PLs and their trading partners. Systech has you covered for DSCSA compliance, including our Verification Router Service (VRS) and Authorized Trading Partner (ATP) support, ensuring full pharma regulatory compliance.
The Falsified Medicines Directive (FMD) requires a system of repositories for pharmaceutical manufacturers to send product information and serial numbers to EU member countries. Systech simplifies this process, helping you connect and ensure pharma regulatory compliance when dispensing medicines across Europe.


Printing and verifying labels for investigational medicinal products (IMPs) used in clinical trials is challenging. But with subject safety, regulatory compliance, track and trace needs and the integrity of clinical trial data on the line, there is no room for labeling errors.
With powerful serialization and traceability functionality we can help you meet global pharmaceutical regulations. Speak with a compliance expert now!
In the rapidly evolving landscape of pharmaceuticals, maintaining regulatory compliance is paramount for pharmacies.
Systech and Vantage discuss what it takes to be compliant by November 2023, the final…
Implementation of credentialing through authorized trading partners is key to product tracing under the DSCSA for 2023.